 I will lead you through the basics of ammonia and fertilizer production. It will tell you not only how ammonia and its derivatives are produced, but will also tell the tale of the downfall of a large fertilizer site as a result of the highly volatile ammonia and fertilizer market. Finally we'll make a trip to the future and explore the possibilities of a 30+ year old chemical plant. This post will give you some basic information about fertilizer and describes the ammonia production process. So here we are... Let's start going...
I will lead you through the basics of ammonia and fertilizer production. It will tell you not only how ammonia and its derivatives are produced, but will also tell the tale of the downfall of a large fertilizer site as a result of the highly volatile ammonia and fertilizer market. Finally we'll make a trip to the future and explore the possibilities of a 30+ year old chemical plant. This post will give you some basic information about fertilizer and describes the ammonia production process. So here we are... Let's start going...Basics
Intensive farming would not be possible without the use of fertilizer. Although it's in fashion to describe the use and production of fertilizer as environmentally polluting, a considerable larger part of the world would die of starvation if fertilizer would not be used. Modern technology makes it possible to distribute fertilizer optimally over farmland, thus greatly reducing the amount of nitrogen and phosphorous dissolving in water around farming areas. Nitrogen is the most important ingredient in farmland to allow crops to grow. The most common nitrogen fertilizers are urea, ammonium nitrate, calcium ammonium nitrate and potassium nitrate. For all these fertilizers, ammonia is the basic raw material.
Technical stuff.
Both fertilizer and ammonia are about nitrogen. Since approximately 80 % of the air we breathe consists of nitrogen, you can imagine that this is a very cheap raw material. The catch is the other crucial raw material needed: hydrogen. Since hydrogen is not freely available, we have to find a way to create it. One of the richest hydrogen sources available is natural gas (methane). One molecule of natural gas consists of one carbon atom and four hydrogen atoms. Other hydrogen resources are "higher" hydrocarbons like propane, butane or even liquid fuels like gasoline. The higher hydrocarbons have the drawback that they contain more carbon atoms per hydrogen atom then natural gas and are therefore less efficient. Hence, natural gas is the most common hydrogen resource. Now the catch should be clear: Natural gas costs money (and quite a lot)!
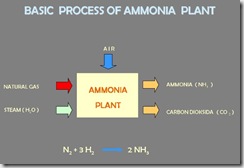
As mentioned, natural gas contains carbon and hydrogen. Since we do not need the carbon, we have to get rid of it and preserve the hydrogen. We can achieve this by adding steam to the natural gas. If the temperature is high enough the steam and natural gas will react and will form carbon dioxide and pure hydrogen (For those more familiar with the exact process: I know that this is horribly oversimplified and not completely true, but it's close enough for this page). The diagram below is the basic process ammonia plant.
Reforming, Purified, Synthesis, and Refrigerant System.
We are now stuck with a mixture of hydrogen and carbon dioxide. The next step will be to separate the carbon dioxide from the hydrogen. The separation towers are the most prominent features of an ammonia plant, as can be seen on the picture to the left (Interesting detail in the picture: Only three of the four towers are used. The fourth one is out of use and serves only as a support for the stacks!)
Now we have to combine the hydrogen with nitrogen to create ammonia. As stated before, the nitrogen is taken from ambient air. The main ingredients in air are nitrogen (~80%) and oxygen (~20%). In an ammonia plant, the air is introduced earlier in the process and the oxygen is used in the hydrogen formation process. So after the carbon dioxide separation-towers we have a mixture of nitrogen and hydrogen.
Regrettably, not only hydrogen and nitrogen are present, but also some impurities. These impurities are "poisons" in the ammonia synthesis, so they have to be removed before the hydrogen and nitrogen is converted to ammonia. The "cleaning" of the hydrogen / nitrogen mixture takes place in several, rather complex, purification processes.
The hydrogen / nitrogen mixture, called syn-gas (from synthesis gas), is introduced in the ammonia reactor. The mixture is only partly converted to ammonia. The gas leaving the reactor is introduced into a condensation process, where the ammonia is liquefied and separated from the unconverted syn-gas. The unconverted syn-gas is recycled to the ammonia reactor. The liquefied ammonia leaving the ammonia plant is of high purity and is either used in subsequent plants or stored in tanks.
Ammonia is always stored as a liquid, generally at atmospheric pressure. At atmospheric pressure, however, the ammonia needs to be refrigerated, since ammonia only liquefies under these conditions at -33 °C. Since most ammonia storage tanks are located in areas where the ambient temperature is above -33 °C, the ammonia in the tanks will have to be cooled continuously. This is generally accomplished by evaporation of ammonia in the tanks. Evaporation costs energy. The evaporation energy is taken from the liquid ammonia, thus keeping its temperature low (If you drive a car on LPG, you probably have seen the effects of evaporation when you were refueling your car: If you disconnect the refueling hose, the area around the connection freezes up). The evaporated ammonia is evacuated from the tanks, compressed, condensed and re-injected in the storage tanks.


which type of catalyst is used now inammonia productionreactors.
we use promoted iron (Fe-base catalyst) for ammonia reactor catalyst.